Global cooling
Mclean, L., Young, I.S. Doherty, M.K., Robertson, D.H.L., Cossins, A.R., Gracey, A. Y., Beynon, R.J. & Whitfield, P.D. (2007) Global cooling: Cold acclimation and the expression of soluble proteins in carp skeletal muscle. Proteomics Proteomics 7, 2667-2681 [PUBMED][PDF]
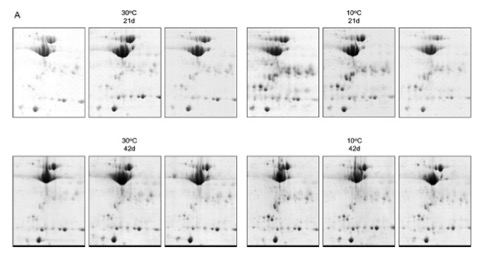
The common carp (Cyprinus carpio) has a well-developed capacity to modify muscle properties in response to changes in temperature. Understanding the mechanisms underpinning this phenotypic response at the protein level may provide fundamental insights into the molecular basis of adaptive processes in skeletal muscle. In this study, common carp were subjected to a cooling regimen and soluble extracts of muscle homogenates were separated by 1-D SDS-PAGE and 2-DE. Proteins were identified using MALDI-TOF-MS and de novo peptide sequencing using LC-MS/MS. The 2-D gel was populated with numerous protein spots that were fragments of all three muscle isoforms (M1, M2 and M3) of carp creatine kinase (CK). The accumulation of the CK fragments was enhanced when the carp were cooled to 10 degrees C. The protein changes observed in the skeletal muscle of carp subjected to cold acclimation were compared to changes described in a previous transcript analysis study. Genes encoding CK isoforms were downregulated and the genes encoding key proteins of the ubiquitin-proteasome pathway were upregulated. These findings are consistent with a specific cold-induced enhancement of proteolysis of CK.