Structure and asparagine deamidation
Rivers J, McDonald L, Edwards IJ, Beynon RJ. (2008) Asparagine deamidation and the role of higher order protein structure. J. Proteome Res. 7, 921-7. [PUBMED][PDF]
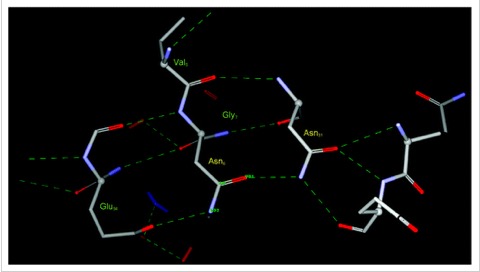
The 'protein world' exhibits additional complexity caused by post-translational modifications. One such process is nonenzymic deamidation of asparagine which is controlled partly by primary sequence, but also higher order protein structure. We have studied the deamidation of an N-terminal peptide in muscle glyceraldehyde 3-phosphate dehydrogenase to relate three-dimensional structure, proteolysis, and deamidation. This work has significant consequences for identification of proteins using peptide mass fingerprinting.